
Experts have hailed the initial results from the University of Oxford’s Covid-19 vaccine as “encouraging”, as data from the latest trial suggests it produces a strong immune response in older people.
The ChAdOx1 nCov-2019 vaccine has been shown to trigger a robust immune response in healthy adults aged 56-69 and people over 70 in phase two clinical trials.
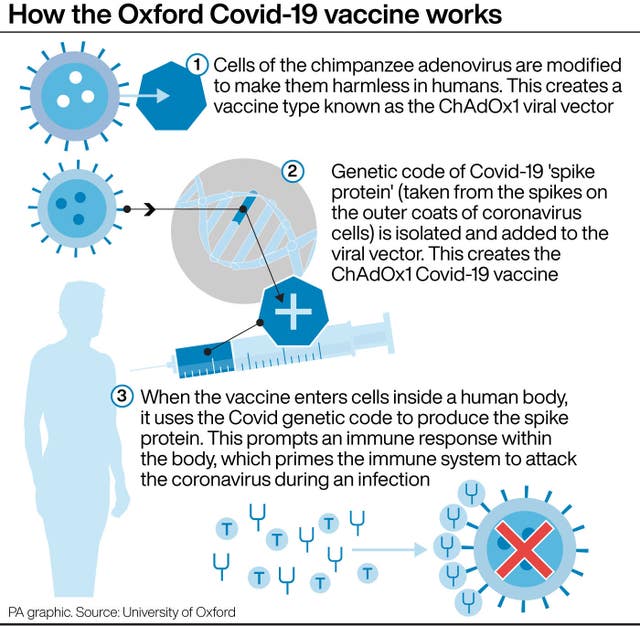
Phase two trials are designed to explore the safety of vaccines and can help scientists learn more about the correct doses while measuring the immune response among people of various ages.
 (PA Graphics)
(PA Graphics)
Professor Deborah Dunn-Walters, chairwoman of the British Society for Immunology Covid-19 and Immunology taskforce, said: “It’s positive to see today’s study published in The Lancet running a phase two trial for the ChAdOx1 nCoV-19 vaccine in an older age group with a median age of 73.
“While this is an ongoing study, the initial results are encouraging.
“The vaccine appears to be well tolerated in all age groups, with older individuals reporting fewer side-effects.”
Phase three trials are ongoing, and the University of Oxford is expected to release data on the efficacy of its vaccine in the coming weeks.
Prof Dunn-Walters added: “At one month after giving two doses of the vaccine, all age groups showed a similar level of antibody response.
“Some age-related differences in the cellular immune response were recorded which require further investigation.
“However, if the immune measures recorded in the phase two part of this study correlate with protection from Sars-Cov-2 (the virus that causes Covid-19), then we would expect positive outcomes from the phase three trial.”
UK authorities have placed orders for 100 million doses of the vaccine – enough to vaccinate most of the population – should it receive regulatory approval.
Experts have cautioned that the results are yet to demonstrate the protective effect of vaccines.
Dr Gillies O’Bryan-Tear, chairman of policy and communications at the Faculty of Pharmaceutical Medicine, said: “We are not there yet – we need to see whether the immune responses in the older patients translates into protection.
Dr Penny Ward, visiting professor in pharmaceutical medicine at King’s College London and chairwoman of the education and standards committee of the faculty, added: “Of note, the population of individuals recruited were older people without significant frailty, living independently in the community.
“It would be useful to extend the study to explore immune response in older, frailer subjects living in care homes.
“However as the vaccine was well tolerated in the study and was effective in similarly aged individuals, this suggests that it could be both well tolerated and effective in older and more infirm individuals in a care home setting.”


Comments: Our rules
We want our comments to be a lively and valuable part of our community - a place where readers can debate and engage with the most important local issues. The ability to comment on our stories is a privilege, not a right, however, and that privilege may be withdrawn if it is abused or misused.
Please report any comments that break our rules.
Read the rules here